What Is An Alkyl Group? Uses
Alkyl Group | What Is An Alkyl Group
In organic chemistry, an alkyl group substituent is an alkane missing one hydrogen. The term alkyl is intentionally unspecific to include many possible substitutions. An acyclic alkyl has the general formula CnH2n+1. A cycloalkyl is derived from a cycloalkane by removal of a hydrogen atom from a ring and has the general formula CnH2n-1. Typically an alkyl is a part of a larger molecule. In structural formula, the symbol R is used to designate a generic (unspecified) alkyl group. The smallest alkyl group is methyl, with the formula CH3−.
Usually, alkyl groups are attached to other atoms or groups of atoms. Free alkyls occur as neutral compounds, as anions, or as cations. The cations are called carbocations. The anions are called carbanions. The neutral alkyl free radicals have no special name. Such species are usually encountered only as transient intermediates, but some are quite stable and can be “put into a bottle”. Typically alkyl cations are generated using super acids, alkyl anions are observed in the presence of strong bases, and alkyl radicals are generated by a photochemical reaction. Alkyls are commonly observed in mass spectrometry of organic compounds.
Alkyl Groups: Reactions Via Proton Loss
Alkyl groups α or γ to a pyridine nitrogen show additional reactions because of the possibility of losing a proton from the carbon atom of the alkyl group which is adjacent to the ring. The ease of proton loss depends on the number, orientation, and nature of the heteroatoms in the ring carrying the alkyl groups as discussed in Section 3.2.3.1. Reactions of this type consist of two essential steps: loss of the proton and then subsequent reaction with an electrophile. For the neutral alkylazines, we distinguish between
- use of a strong base which removes the proton completely before addition of electrophile;
- use of a weaker base which sets up a pre-equilibrium giving traces of reactive anion which reacts with the electrophile and is then replenished by the equilibrium; and
- use of an acid catalyst which may produce small amounts of the tautomeric methylene form (e.g., 647) which then reacts as an enamine at the side-chain carbon.
Classification Of Carbon Atoms
Carbons have a special terminology to describe how many other carbons they are attached to.
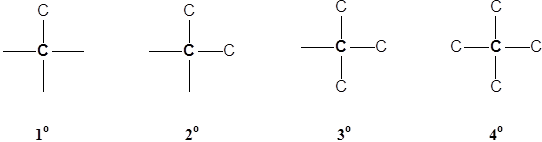
- Primary carbons (1o) attached to one other C atom
- Secondary carbons (2o) are attached to two other C’s
- Tertiary carbons (3o) are attached to theree other C’s
- Quaternary carbons (4o) are attached to four C’s
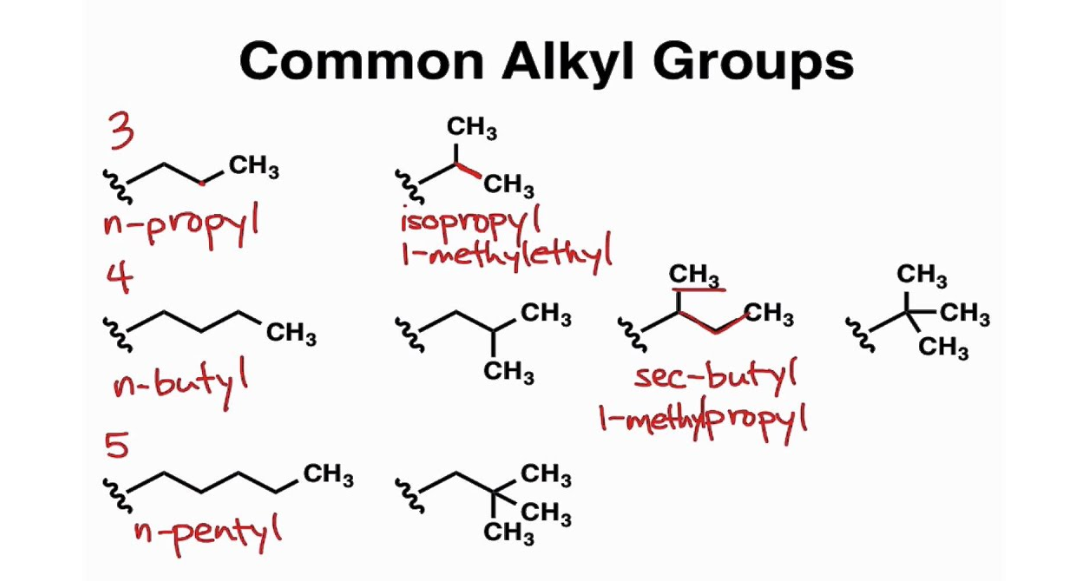
Naming An Alkyl Group
In organic chemistry, there’s a distinct set of rules you must follow when naming compounds. The nomenclature, or rulebook, used for naming molecules is called IUPAC, or the International Union of Pure and Applied Chemistry. If we start with alkane nomenclature you will see how easy it is to name alkyl molecules. Just remember that alkane and alkyl are two different functional groups based on the removal of one hydrogen atom.
With all functional groups, the first process in naming involves determination of the prefix. This is based on how many carbon atoms are present in the longest chain of a compound. Also, prefix is based on carbon count. Once you determine your prefix name, next up is the suffix ending. In the case of alkane functional groups, the suffix ending would be, ‘-ane.’ However, we are interested in the alkyl group. If an alkyl group is present, you simply change the alkane ending of ‘-ane’ to ‘-yl.’ Let’s look at this hexane molecule as an example.
Alkyl Functional Group
Alkyl Halide
Alkyl halides [haloalkanes] consist of an alkyl group attached to a halogen: F, Cl, Br, I. Chloro, bromo and iodo alkyl halides are often susceptible to elimination and/or nucleophilic substitution reactions.
Alkane
Alkyl, and occasionally aryl (aromatic) functions are represented by the R- Methyl: CH3–
Ethyl: CH3CH2–
Propyl: CH3CH2CH2–
Isopropyl: (CH3)2CH–
Phenyl: C6H5– etc.
Primary Alcohol
Primary alcohols have an -OH function attached to an R-CH2- group. Primary alcohols can be oxidised to aldehydes and on to carboxylic acids. (It can be difficult to stop the oxidation at the aldehyde stage.) Primary alcohols can be shown in text as: RCH2OH
Aldehyde
Aldehydes have a hydrogen and an alkyl (or aromatic) group attached to a carbonyl function. Aldehydes can be shown in text as: RCHO Aldehydes are easily oxidised to carboxylic acids, and they can be reduced to primary alcohols. Aldehydes can be distinguished from ketones by giving positive test results with Fehlings solution (brick red precipitate) or Tollens reagent (silver mirror). Aldehydes give red-orange precipitates with 2,4-dinitrophenyl hydrazine.
Uses In everyday life
The word root alkyl is encountered in several contexts. Alkylation is an important operation in refineries, for example in the production of high-octane gasoline. Alkylating antineoplastic agents refer to a class of compounds that are used to treat cancer. In such case, the term alkyl is used loosely. For example, nitrogen mustards are well-known alkylating agents, but they are more complex than a mere hydrocarbon. In chemistry, alkyl refers to a group, a substituent, that is attached to other molecular fragments. For example, alkyl lithium reagents have the empirical formula Li(alkyl), where alkyl = methyl, ethyl, etc. A dialkyl ether is an ether with two alkyl groups, e.g., diethyl ether (O(C2H5)2). Alkyl also refers to an active ingredient in green algae preventatives used in swimming pools.


