What Is A Hydroxyl Group? Properties
Hydroxyl Group | What Is A Hydroxyl Group
A hydroxyl group increases the ECL value of a fatty acid greatly, especially on polar columns, and if the hydroxyl group is derivatized, the nature of the derivative has a profound effect on the elution profile for different isomers.
The hydroxyl group is a functional group consisting of a hydrogen atom covalently bonded to an oxygen atom. The hydroxyl group is denoted by -OH in chemical structures and has a valence charge of -1. The hydroxyl radical is very reactive, so it quickly reacts with other chemical species. Hydroxyl radicals can cause DNA and cell damage.
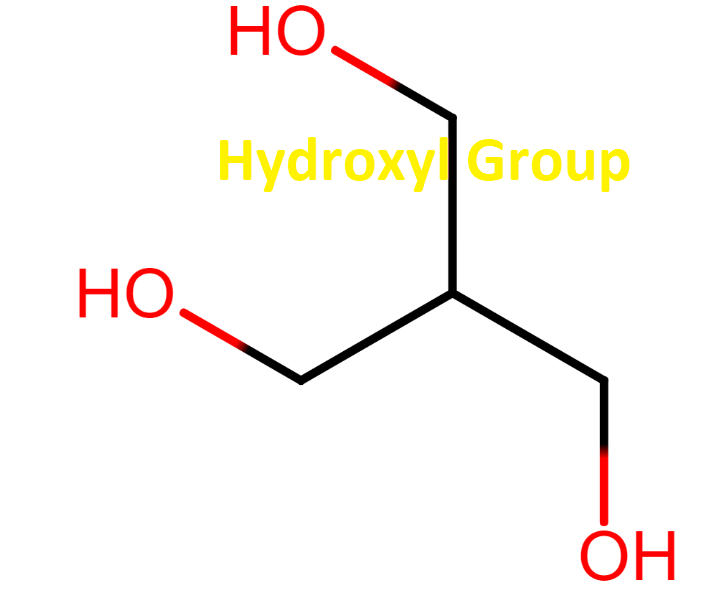
Chemical Formula and Structure Of The Hydroxyl Group
A hydroxyl group is composed of one hydrogen atom bonded to one oxygen atom. Its chemical formula is written as either -OH or HO-. The ‘-‘ represents the carbon to which the hydroxyl group is bonded.
Hydroxyl Functional GroupThe R in the structural formula stands for the carbon backbone of the organic molecule to which the hydroxyl attaches.
Have you ever used rubbing alcohol to clean a minor cut or scrape? Have you ever seen people drink an adult beverage, such as wine or beer? If you answered yes to either of these questions, then you already have some familiarity with hydroxyl groups.
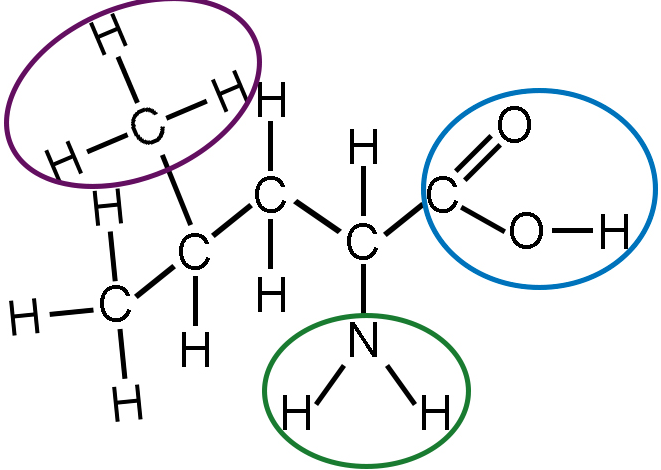
Hydroxyl groups are a class of organic functional groups. In organic chemistry, carbon-containing compounds are grouped according to the arrangement of certain atoms in the molecule. These arrangements are called functional groups, so named because they make the compound function, or react, in a specific way. When a hydroxyl group is the dominant functional group in an organic compound, that compound behaves as an alcohol.
Functional Groups in Organic Molecules
To further explore the specifics of a hydroxyl group, let’s go back to our basic understanding of an organic molecule. Organic molecules are carbon-based and also may contain oxygen, hydrogen, nitrogen, sulfur, and/or phosphorus. Structurally, these molecules are composed of two main parts.The first part is the carbon backbone, in which the carbon atoms are bonded together forming a carbon backbone.
The second are the functional groups, which are small groups of atoms, such as hydrogen and oxygen, that are bonded to the carbon backbone. Functional groups are so named because they function as the chemically reactive area of the molecule.
The hydroxyl group (-OH) is one example of a functional group. When hydroxyl groups are the primary functional group bonded to carbon backbones, the resulting molecules are alcohols. Here we see the structural formula for the organic molecule ethanol (a type of alcohol) with the hydroxyl group on the far right.Methanol, isopropyl alcohol, and propanol are additional examples of alcohols containing the hydroxyl group.Carbohydrate molecules, or sugars, have hydroxyl groups, too. However, sugars also contain another important functional group, called the carbonyl group (-CO), that alcohols don’t have. This is what distinguishes sugars from alcohols. Looking at the structure of a sugar called glucose, you can see that there are hydroxyl groups on each side of both examples.
Hydroxyl Versus Hydroxy
The terms “hydroxyl” and “hydroxy” tend to be used interchangeably, but they don’t technically mean the same thing. The term hydroxyl means the radical OH. The functional group -OH is more properly called a hydroxy group. Further, the [OH–] anion, which is called hydroxide, consists of a hydroxy group.
Related Biology Terms
- Carboxyl group – A carbon doubled bonded to an oxygen and also bonded to a hydroxyl group.
- Carbonyl group – A carbon double bonded to an oxygen and any other molecules, including more carbons.
- Electronegativity – The attraction that an atom has for electrons, compared to the other types of atoms that it shares electrons with in covalent bonds.
- Polarity – The property of a molecule that arises from the stable differentiation of electrical poles across a molecule or part of a molecule.
Hydroxyl Group Properties
Water, alcohols, carboxylic acids, and many other hydroxy-containing compounds can be deprotonated readily. This behavior is rationalized by the disparate electro-negativities of oxygen and hydrogen. Hydroxy-containing compounds engage in hydrogen bonding, which causes them to stick together, leading to higher boiling and melting points than found for compounds that lack this functional group. Organic compounds, which are often poorly soluble in water, become water-soluble when they contain two or more hydroxy groups, as illustrated by sugars and amino acid.
Difference Between Hydroxyl and Alcohol
Organic compounds are molecules that are essentially composed of C and H atoms. There can be other atoms such as O and N bonded to these molecules. Alcohol is such a molecule made out of C, H and O atoms. The characteristic feature of alcohols is the presence of a hydroxyl (-OH) attached to an alkyl. A functional group is a group of atoms that decide the properties and reactions of a particular molecule. Hydroxyl is a functional group present in organic and inorganic compounds such as alcohols.
The acidity or basicity of the molecule can vary depending on the location of the hydroxyl in the molecule. But according to the IUPAC nomenclature (the method of naming compounds introduced by the International Union of Pure and Applied Chemistry – IUPAC), the term hydroxyl stands for the hydroxyl radical. However, we still use this term for both hydroxyl and hydroxyl radical. The main difference between hydroxyl and alcohol is that a hydroxyl is a functional group whereas alcohol is an organic compound.


